3D Bioprinting of a Bionic Pancreas
Project: “3D Bioprinting of scaffolds using live pancreatic islets or insulin-producing cells to create a bionic pancreas,” funded by the National Centre for Research and Development under the “Prevention and Treatment of Civilization Diseases” STRATEGMED program.
Implementation period: 01.01.2017 – 30.06.2021.
Total project cost: 24 709 750,00 PLN
NCBR funding: 23 143 024,00 zł
Diabetes is a chronic disease of growing economic and social significance. he number of people with diabetes has tripled in the last 20 years, currently affecting 463 million people worldwide. The World Health Organization (WHO) forecasts that these numbers will double within the next 10 years. As much as 10% of global health expenditures (€646 billion) are allocated to diabetes.
In Poland, approximately 3 million people suffer from diabetes, with 200,000 of them being patients with type 1 diabetes.
Individuals living with type 1 diabetes cannot produce insulin, and their lives depend on daily injections. Although chronic insulin therapy is a solution that allows many people to function, it is a temporary alternative. Insulin therapy does not halt the progression of the disease and does not guarantee the absence of certain complications. The only way to completely cure diabetes is through a whole pancreas or pancreatic islet transplant from a donor. However, in Europe, fewer than 200 pancreas transplants are performed annually (out of approximately 400,000 in need) due to organ shortages. In the case of islet transplantation, the method only provides short-term effectiveness and requires periodic repetitions.
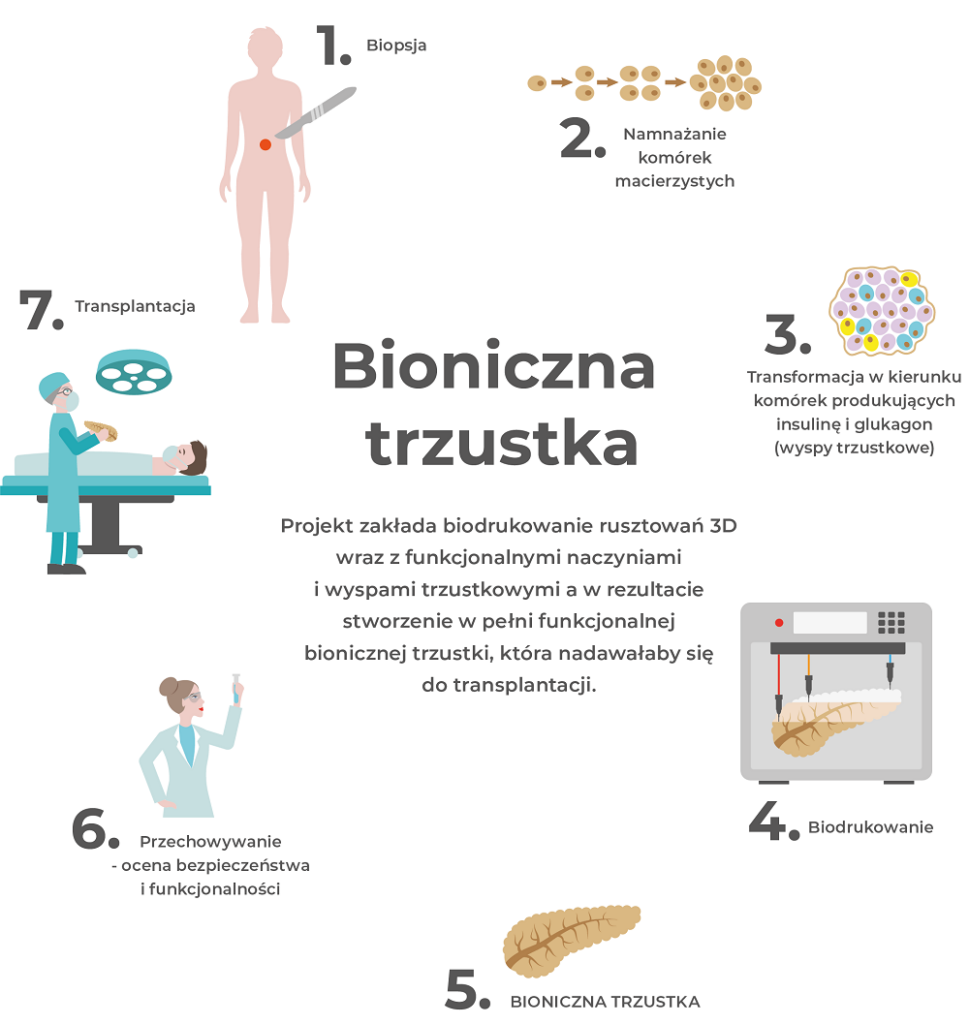
Polbionica aims to address both problems. Our innovative solution is a fully functional, bionic organ printed in 3D using our proprietary bioinks and pancreatic islets, and subsequently, the patient’s own stem cells. Our solution fills the gap between organ shortages and transplantation needs, providing long-term independence from exogenous insulin.

A Tailored Solution
Transplantation of a bionic pancreas will prevent the development of complications in individuals suffering from diabetes. Initially, the bionic pancreas transplant program will target those patients who could potentially qualify for a transplant of a real organ. This group includes individuals with severe complications, in whom a bionic pancreas transplant can realistically halt the onset of secondary complications. Printing a bionic pancreas not only offers the opportunity to save human health and lives but also reduces healthcare expenditures associated with treating diabetic patients. Currently, these expenditures amount to 2.5 billion Polish złoty annually, accounting for 9% of all healthcare expenditures. It’s worth noting that one-fifth of this sum is allocated solely for the treatment of diabetic complications.
As a result of implementing the project, the financial resources needed to treat patients with complications will significantly decrease, along with future costs of immunosuppressive therapy.
Our world’s first bionic pancreas with full vascularization has successfully passed preclinical tests and is ready to demonstrate its effectiveness in clinical trials. The key to our innovation is providing a physiologically appropriate environment for pancreatic islets to ensure they remain healthy and functional. The bionic pancreas can be 3D bioprinted “on demand” and transplanted with minimal risk of complications. It has the potential to change the landscape of treating patients with type 1 diabetes and those with chronic pancreatitis.
Success of Polish Scientists
In March 2019, a multidisciplinary team of scientists from the Foundation for Research and Development of Science, led by Dr. Michał Wszoła, achieved the world’s first bioprinting of a bionic pancreas with a complete vascular system. In the subsequent stage, the bionic pancreas was connected to a special device developed by a team of engineers—a bioreactor—that supplies the printed vascular system with oxygenated fluid and nutrients.
Polbionica, a spin-off company established by the Foundation for Research and Development of Science, leverages its scientific achievements to prepare the bionic pancreas project for the clinical phase and ultimately for commercialization, which will mean its implementation into medical practice in Poland and worldwide.
Our unique project also involved and continues to involve major scientific, medical, and technical institutions. The project was carried out by the BIONIC Consortium, established by the initiative of the Foundation for Research and Development of Science, which includes:
- The M. Nencki Institute of Experimental Biology PAN, with the team led by Prof. Agnieszka Dobrzyń
- The Faculty of Materials Science and Engineering at Warsaw University of Technology, under the direction of Prof. Wojciecha Święszkowskiego
- The Biostructure Center at the Medical University of Warsaw, headed by Prof. Artura Kamińskiego
- The MediSpace Medical Center Sp. z o.o.
The BIONIC Consortium received funding for the project titled “3D Bioprinting of scaffolds using live pancreatic islets or insulin-producing cells to create a bionic pancreas” under the STRATEGMED program “Prevention and Treatment of Civilization Diseases”.
The Future Begins Today
We believe that the future of organ printing is now.
Our innovative, fully functional 3D bioprinted bionic pancreas, based on bioprinted scaffolds, functional vessels, and pancreatic islets, is ready for clinical trials. This first-of-its-kind bioprinted organ has the ability to produce insulin, glucagon, and C-peptide. Our technology directly addresses the growing transplantation challenges—organ shortages and the low efficacy of pancreatic islet transplantation.
Our proprietary bioinks provide an appropriate physiological environment and vascularization, ensuring nutrients and proper oxygenation for the pancreatic islets. The vascular system with bioprinting does not require complicated surgery as it is connected to an artery that is 80% smaller than currently used vessels and does not need to be introduced into the abdominal cavity.
We have received a scientific recommendation from the European Medicines Agency (EMA) regarding ATMP classification (EMA/CAT/508907/2020), indicating that the bionic pancreas can be transplanted under the Hospital Exemption Rule and classifying the bionic pancreas as a CATMP.
Further development of organ manufacturing technology would significantly impact public health, largely eliminating the problem of diseases requiring transplantation, reducing treatment costs, the need for social care, and job absenteeism, while improving the quality of life for patients.
The bioprinted bionic pancreas is cheaper than traditional islet transplantation, provides long-term independence from insulin, and eliminates the daily burden of disease management. Our solution is specifically dedicated to patients with complications.