Z nauki do zdrowia
Drukujemy bioniczną trzustkę!
Polbionica jest innowacyjną firmą biotechnologiczną rozwijającą pionierski w skali świata projekt biodruku 3D trzustki – w pełni funkcjonalnego narządu z żywych komórek skutecznie produkującego insulinę i glukagon. Przeszczep bionicznej trzustki pacjentowi z cukrzycą typu 1 pozwoli na wyleczenie pacjenta oraz jego powrót do funkcjonowania w społeczeństwie jako w pełni zdrowego człowieka.
Naszą misją jest znacząca poprawa jakości życia pacjentów diabetyków na całym świecie poprzez wprowadzenie drukowania 3D bionicznej trzustki do praktyki klinicznej. Wyniki badań uwodniły, że nasza bioniczna trzustka to organ, który przywróci zdolność organizmu do regulowania poziomu cukru we krwi.

W 2020 roku Polbionica otrzymała certyfikat Komisji Europejskiej THE SEAL OF EXCELLENCE IN HORIZON 2020

Realizacja tak innowacyjnego i unikatowego w skali światowej projektu badawczego jak biodrukowanie 3D bionicznej trzustki jest możliwe dzięki zaangażowaniu najważniejszych medycznych i technicznych instytucji naukowych w Polsce. Planowane wdrożenie kliniczne będzie realizowane przez konsorcjum, w którego skład oprócz Polbionica wejdą: Fundacja Badań i Rozwoju Nauki, Centrum Zaawansowanych Technologii Uniwersytetu im. Adama Mickiewicza, Centrum Medyczne MediSpace oraz Warszawski Uniwersytet Medyczny przy współpracy z University of Chicago i University of Leiden w Holandii.

Prof. dr hab. n. med. Michał Wszoła
Prezes Zarządu Polbionica
Partnerzy






Projekty
- biodrukowanie 3D bionicznej trzustki
- opracowanie biotuszy do biodrukarki 3D
- bioreaktor
- AURES® – automatyczny resuscytator
O nas
Drukujemy organy. To nie jest fikcja. To się dzieje naprawdę!
Polbionica zajmuje się komercjalizacją wyników badań przedklinicznych i klinicznych opartych na biodruku 3D bionicznej trzustki.
Zadzwoń
+48 518 785 622
Napisz
contact@polbionica.com
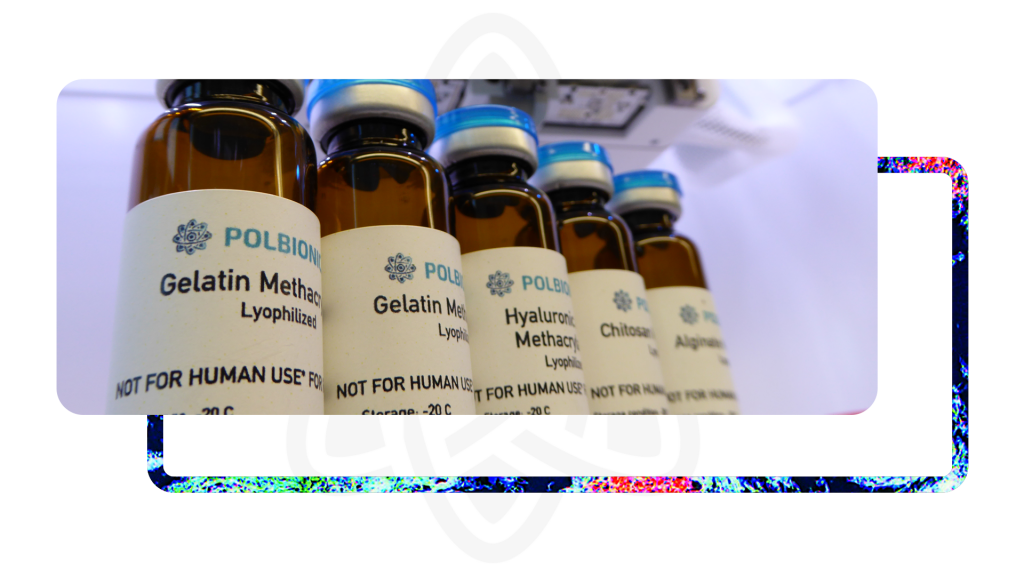
Nasze produkty
Pozytywne rezultaty naszych zaawansowanych prac B+R, dopracowana własna produkcja biomateriałów i ich codzienne stosowanie spowodowały, że z satysfakcją chcemy podzielić się naszymi najlepszymi doświadczeniami.
Zapraszamy do zapoznania się z ofertą oraz do zakupu wyselekcjonowanych produktów i sprzętu linii produktowej TINTBIONICTM.
Uzupełnieniem jest nasza unikalna oferta dedykowanych usług związanych z biodrukiem 3D. Propozycja usług jest kompetentną odpowiedzią na często zgłaszane pytania Klientów.
TINTBIONICTM jest znakiem towarowym należącym do Polbionica.